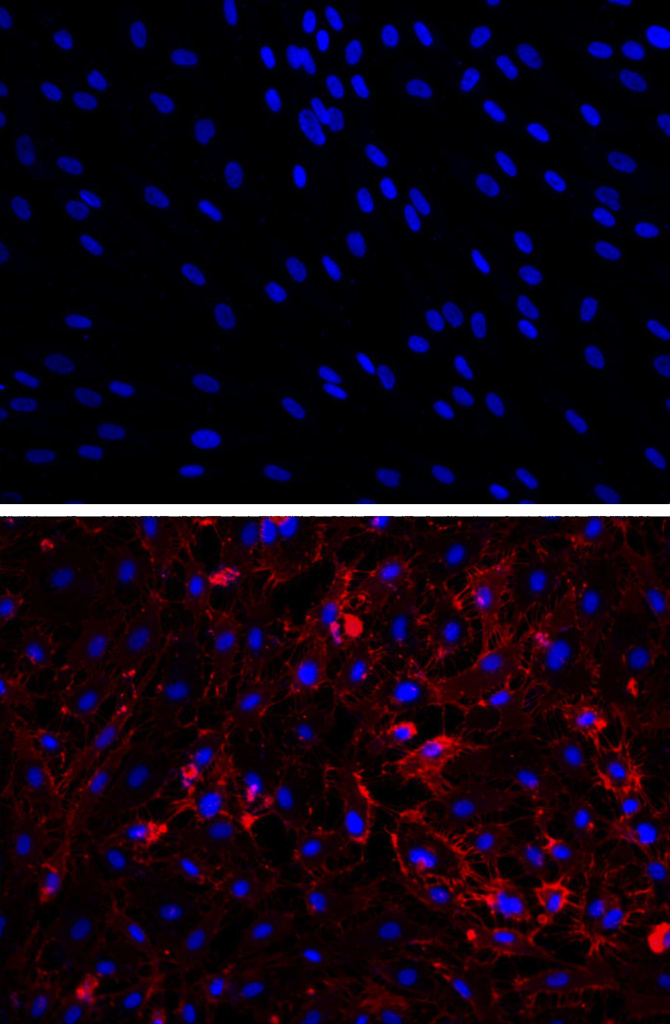
NEWSWISE — HOUSTON — ( Nov. 7, 2014 ) — By transforming human scar cells into blood vessel cells, scientists at Houston Methodist may have discovered a new way to repair damaged tissue. The method, described in an upcoming issue of Circulation (early online), appeared to improve blood flow, oxygenation, and nutrition to areas in need.
Cardiovascular scientists at Houston Methodist, with colleagues at Stanford University and Cincinnati Children’s Hospital, learned that fibroblasts — cells that causes scarring and are plentiful throughout the human body — can be coaxed into becoming endothelium, an entirely different type of adult cell that forms the lining of blood vessels.
“To our knowledge, this is the first time that trans-differentiation to a therapeutic cell type has been accomplished with a small molecules and proteins,” said Houston Methodist Research Institute Department of Cardiovascular Sciences Chair John Cooke, M.D., Ph.D., the study’s principal investigator. “In this particular case, we’ve found a way to turn fibroblasts into ‘shapeshifters’ nearly on command.”
Cooke said the regenerative medicine approach provides proof-of-concept for a small molecule therapy that could one day be used to improve the healing of cardiovascular damage or other injuries.
Other research groups have managed to generate endothelial cells cells using infectious virus particles specially engineered to deliver gene-manipulating DNA to cells. The DNA encodes proteins called transcription factors to alter gene expression patterns in such a way that cells behave more like endothelial cells.
“There are problems with using viruses to transfer genes into cells,” Cooke said. “This gene therapy approach is more complicated, and using viral vectors means the possibility of causing damage to the patient’s chromosomes. We believe a small-molecule approach to transforming the cells will be far more feasible and safer for clinical therapies.”
The new method described by Cooke and his coauthors starts with exposing fibroblasts to poly I:C (polyinosinic:polycytidylic acid), a small segment of double-stranded RNA that binds to the host cell receptor TLR3 (toll-like receptor 3), tricking the cells into reacting as if attacked by a virus. Cooke and coauthors reported to Cell in 2012 that fibroblasts’ response to a viral attack — or, in this case, a fake viral attack — appears to be a vital step in diverting fibroblasts toward a new cell fate. After treatment with poly I:C, the researchers observed a reorganization of nuclear chromatin, allowing previously blocked-off genes to be expressed. The fibroblasts were then treated with factors, such as VEGF, that are known to compel less differentiated cells into becoming endothelial cells.
Cooke and his colleagues reported to Circulation that about 2 percent of the fibroblasts were transformed from fibroblasts into endothelial cells, a rate comparable to what other research groups have accomplished using viruses and gene therapy. But Cooke said preliminary, as-yet-unpublished work by his group suggests they may be able to achieve transformation rates as high as 15 percent.
“That’s about where we think the yield of transformed cells needs to be,” Cooke said. “You don’t want all of the fibroblasts to be transformed — fibroblasts perform a number of important functions, including making proteins that hold tissue together. Our approach will transform some of the scar cells into blood vessel cells that will provide blood flow to heal the injury.”
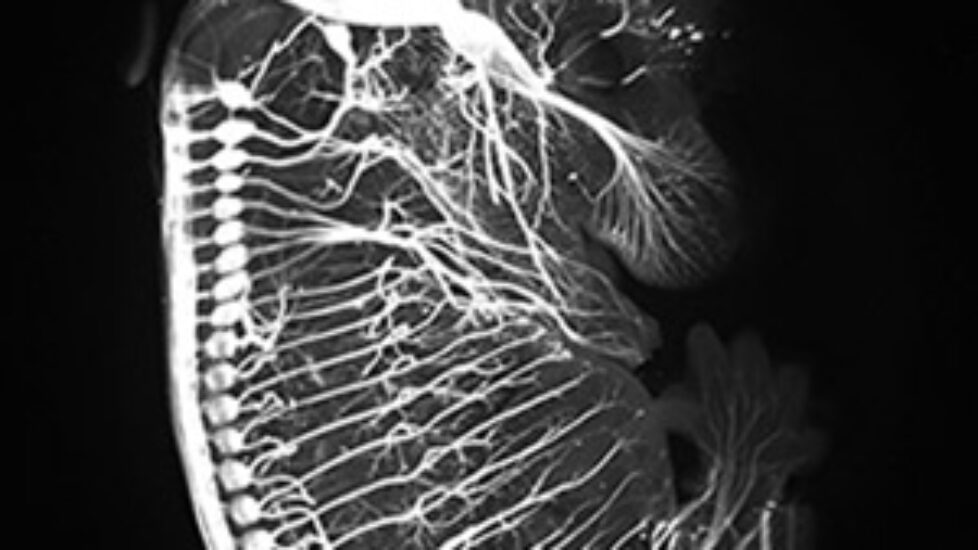
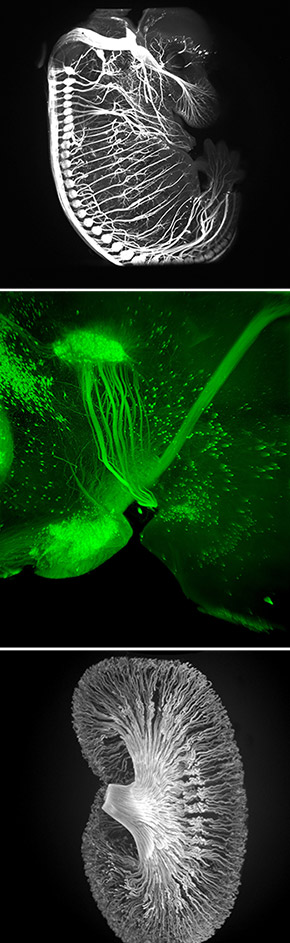
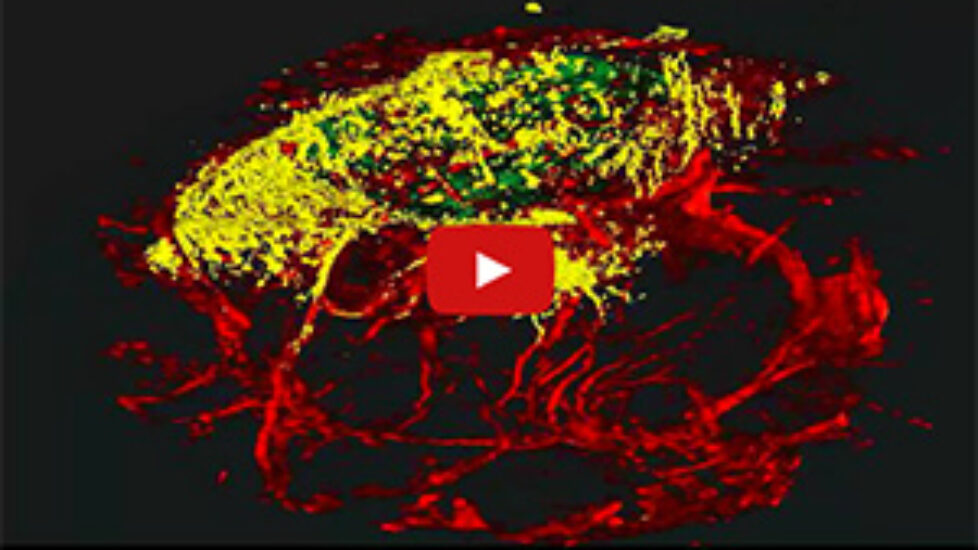
In a second part of the study, the scientists introduced the transformed human cells into immune-deficient mice that had poor blood flow to their hind limbs. The human blood vessel cells increased the number of vessels in the mouse limb, improving circulation.
“The cells spontaneously form new blood vessels — they self assemble,” Cooke said. “Our transformed cells appear to form capillaries in vivo that join with the existing vessels in the animal, as we saw mouse red blood cells inside the vessels composed of human cells.”
Cooke, who is also the director of the Houston Methodist Center for Cardiovascular Regeneration, said that figuring out how to manipulate adult cells of one type into becoming a completely different type of cell will be an important part of the development of regenerative medicine as a scientific and clinical field. Humans are generally unable to regenerate heavily damaged tissue, whereas other animals, such as some newts and flat worms, can regenerate entire lost limbs — even entire heads.
“It is likely that modifications of this small molecule approach may be used to generate other body cells of therapeutic interest,” Cooke said. “What we are seeing is evidence of the fluidity of cell fate with the proper stimulation. If we can understand the underlying pathways and how to manipulate them, we may very well learn how reawaken primordial mechanisms for regeneration that are active in lower vertebrates such as newts.”
Cooke said more animal model studies are needed before his group begins clinical trials.
“One of the next steps will be to see if we can rescue an animal from an injury,” Cooke said. “We want to know if the therapy enhances healing by increasing blood flow to tissues that may have been damaged by a loss of blood because of ischemia.”
To speak with John Cooke, please contact David Bricker, Houston Methodist, at 832-667-5811 or dmbricker@houstonmethodist.org.

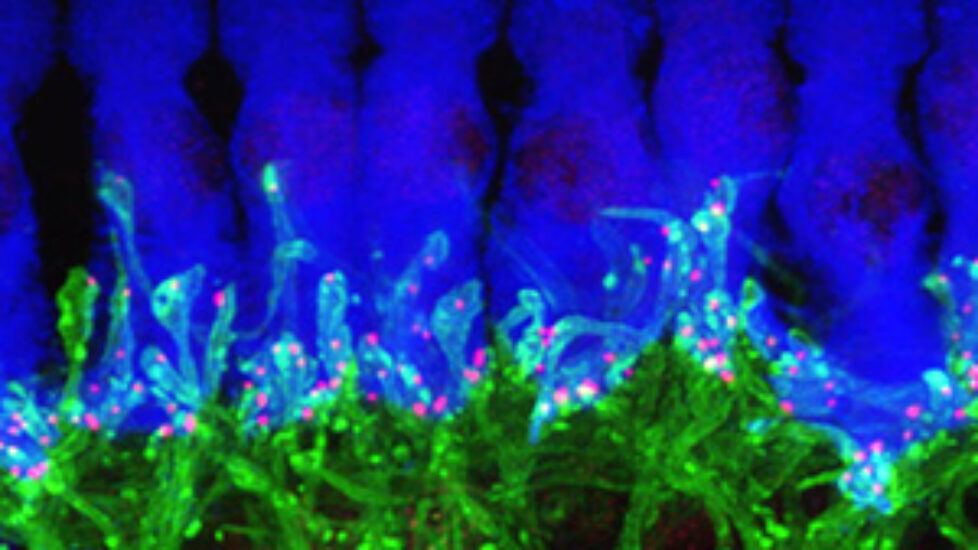
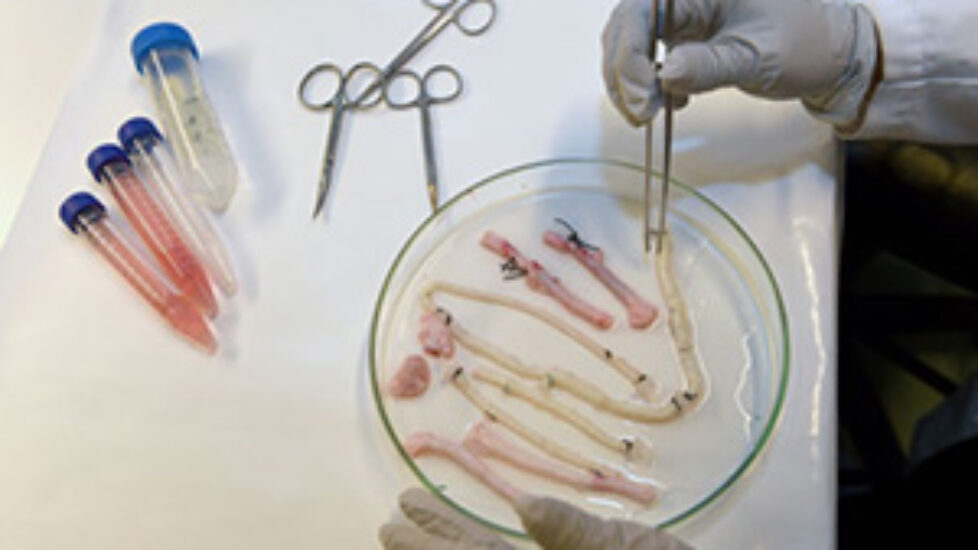
 The technology for creating new tissues from stem cells has taken a giant leap forward. Two tablespoons of blood are all that is needed to grow a brand new blood vessel in just seven days. This is shown in a new study from Sahlgrenska Acadedmy and Sahlgrenska University Hospital published in EBioMedicine.
The technology for creating new tissues from stem cells has taken a giant leap forward. Two tablespoons of blood are all that is needed to grow a brand new blood vessel in just seven days. This is shown in a new study from Sahlgrenska Acadedmy and Sahlgrenska University Hospital published in EBioMedicine.  Professors Sumitran-Holgersson and Olausson have published a new study in EBioMedicine based on two other transplants that were performed in 2012 at Sahlgrenska University Hospital. The patients, two young children, had the same condition as in the first case – they were missing the vein that goes from the gastrointestinal tract to the liver.
Professors Sumitran-Holgersson and Olausson have published a new study in EBioMedicine based on two other transplants that were performed in 2012 at Sahlgrenska University Hospital. The patients, two young children, had the same condition as in the first case – they were missing the vein that goes from the gastrointestinal tract to the liver.